What is the enthalpy change (in kJ) of a chemical reaction that raises the temperature of 250.0 mL of solution having a density of 1.25 g 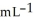 by 10.2 °C? (The specific heat of the solution is 3.733 J
by 10.2 °C? (The specific heat of the solution is 3.733 J 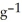
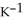 .)
.)
A) -7.43 kJ
B) -12.51 kJ
C) 8.20 kJ
D) -11.9 kJ
E) 6.51 kJ
Correct Answer:
Verified
Q106: Calculate the work,w,gained or lost by the
Q111: For a particular process that is carried
Q131: The specific heat capacity of methane gas
Q132: The specific heat capacity of liquid water
Q133: How much heat is absorbed when 50.00
Q134: A 5.00 g sample of liquid water
Q136: Hydrogen peroxide decomposes to water and oxygen
Q138: The specific heat capacity of liquid mercury
Q139: The specific heat capacity of solid copper
Q140: A 50.0 g sample of liquid water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents