Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + 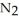 (g) →
(g) → 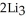 N(s)
N(s)
How many moles of lithium are needed to produce 0.40 mol of 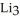 N when the reaction is carried out in the presence of excess nitrogen?
N when the reaction is carried out in the presence of excess nitrogen?
A) 0.13
B) 1.2
C) 0.20
D) 0.067
E) 2.4
Correct Answer:
Verified
Q101: Which of the compounds H2C2O4,Ca(OH)2,KOH,and HI behave
Q102: In an acid-base neutralization reaction,38.74 mL of
Q119: Which one of the following compounds behaves
Q149: What is the oxidation number of the
Q166: Automotive air bags inflate when sodium azide
Q168: Automotive air bags inflate when sodium azide
Q169: How many millilitres of 0.152 mol L-1
Q170: A solution is prepared by mixing 70.0
Q174: Magnesium burns in air with a dazzling
Q176: When 280. mL of 1.50 × 10-4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents