Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ) 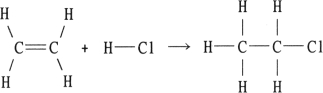
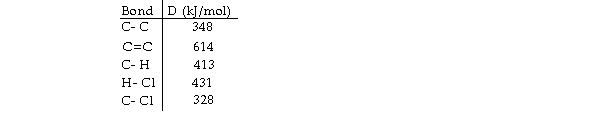
A) - 44
B) - 38
C) 304
D) 38
E) 2134
Correct Answer:
Verified
Q64: The formal charge on sulfur in SO42-
Q65: Using the table of bond dissociation energies,
Q66: Which of the following would have to
Q67: What is the maximum number of double
Q68: A nonpolar bond will form between two
Q70: Based on the octet rule, iodine most
Q71: Which of the following would have to
Q72: The ion ICI4- has _ valence electrons.
A)
Q73: Determining lattice energy from Born- Haber cycle
Q74: How many single covalent bonds must a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents