The formal charge on sulfur in SO42- is , where the Lewis structure of the ion is: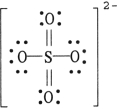
A) 0
B) - 4
C) - 2
D) +4
E) +2
Correct Answer:
Verified
Q59: Why don't we draw double bonds between
Q60: Give the electron configuration of Cu2+.
Q61: For a given arrangement of ions, the
Q62: How many unpaired electrons are there in
Q63: Electronegativity _ from left to right within
Q65: Using the table of bond dissociation energies,
Q66: Which of the following would have to
Q67: What is the maximum number of double
Q68: A nonpolar bond will form between two
Q69: Using the table of bond dissociation energies,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents