How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 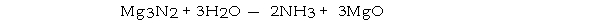
A) 0.0378
B) 0.113
C) 0.429
D) 4.57
E) 0.0756
Correct Answer:
Verified
Q65: Write the balanced equation for the reaction
Q67: The combustion of ammonia in the presence
Q68: When the following equation is balanced, the
Q70: The combustion of carbon disulfide in the
Q71: When the following equation is balanced, the
Q73: A compound is composed of only C,
Q75: A 2.25- g sample of magnesium nitrate,
Q76: Under appropriate conditions, nitrogen and hydrogen undergo
Q124: Determine the mass percent (to the hundredths
Q177: Complete and balance the following reaction, given
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents