In the nuclear transmutation,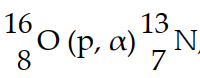 , what is the bombarding particle?
, what is the bombarding particle?
A) a phosphorus nucleus
B) a gamma photon
C) an alpha particle
D) a beta particle
E) a proton
Correct Answer:
Verified
Q4: In the nuclear transmutation represented by
Q5: The mass of a proton is 1.00728
Q6: The basis for the carbon- 14 dating
Q7: Which type of radioactive decay results in
Q8: A rock contains 0.313 mg of lead-
Q10: What is required for a nuclear transmutation
Q11: 210Pb has a half- life of 22.3
Q12: At approximately what number of protons, or
Q13: If we start with 1.000 g of
Q14: The mass of a proton is 1.673
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents