The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy (in J) of a 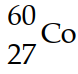 nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.)
A) 8.206 × 10- 11
B) 9.117 × 10- 28
C) 4.940 × 10- 13
D) 2.735 × 10- 16
E) 2.735 × 10- 19
Correct Answer:
Verified
Q1: Which of these nuclides is most likely
Q2: In the nuclear transmutation represented by
Q3: If we start with 1.000 g of
Q4: In the nuclear transmutation represented by
Q6: The basis for the carbon- 14 dating
Q7: Which type of radioactive decay results in
Q8: A rock contains 0.313 mg of lead-
Q9: In the nuclear transmutation, Q10: What is required for a nuclear transmutation Q11: 210Pb has a half- life of 22.3![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents