The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is (Amu) 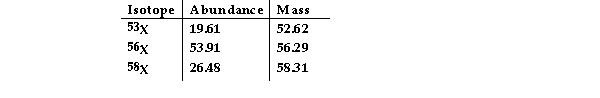
A) 57.23
B) 56.11
C) 33.33
D) 56.29
E) 55.74
Correct Answer:
Verified
Q60: Which one of the following basic forces
Q61: The atomic mass unit is presently based
Q62: Which pair of elements below should be
Q63: The mass number of an atom of
Q64: Different isotopes of a particular element contain
Q66: The species contains 16 neutrons.
A). 36Cl
B). 31P
C).
Q67: Which combination of protons, neutrons, and electrons
Q68: There are electrons, protons, and neutrons in
Q69: In the Rutherford nuclear- atom model, .
A)
Q70: Gravitational forces act between objects in proportion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents