The equilibrium position corresponds to which letter on the graph of G vs f (course of reaction) below?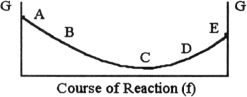
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q1: For a given reaction, OH = +35.5
Q2: OS is positive for the reaction _.
A)
Q4: In the Haber process, ammonia is synthesized
Q5: A reaction that is spontaneous as written
Q6: ΔS is negative for the reaction .
A)
Q7: The value of ΔG° at 100.0 °C
Q8: With thermodynamics, one cannot determine .
A) the
Q9: The entropy of the universe is .
A)
Q10: The thermodynamic quantity that expresses the degree
Q11: For the reaction ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents