Consider the reaction: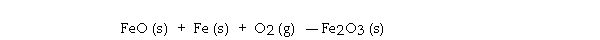
Given the following table of thermodynamic data, 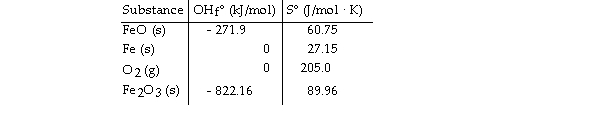
Determine the temperature (in °C) above which the reaction is nonspontaneous.
A) 756.3
B) 2439
C) This reaction is spontaneous at all temperatures.
D) 618.1
E) 1235
Correct Answer:
Verified
Q54: Consider the reaction: Q55: Use the table below to answer the Q56: Calculate OG○for the autoionization of water at Q57: Use the table below to answer the Q58: Which one of the following processes produces Q60: Find the temperature above which a reaction Q61: Use the table below to answer the Q62: Use the table below to answer the Q63: Use the table below to answer the Q64: Use the table below to answer the![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents