Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 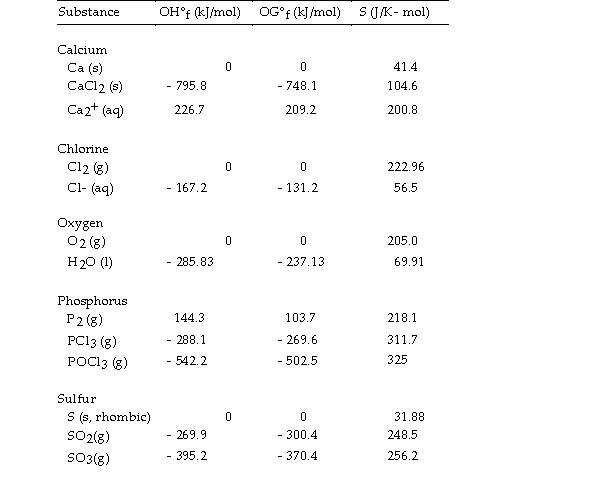
-The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is kJ/mol.
is kJ/mol.
A) - 269.9
B) +11.6
C) - 11.6
D) +269.9
E) +0.00
Correct Answer:
Verified
Q50: Use the table below to answer the
Q51: For the reaction Q52: Use the table below to answer the Q53: Consider a pure crystalline solid that is Q54: Consider the reaction: Q56: Calculate OG○for the autoionization of water at Q57: Use the table below to answer the Q58: Which one of the following processes produces Q59: Consider the reaction: Q60: Find the temperature above which a reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

