Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 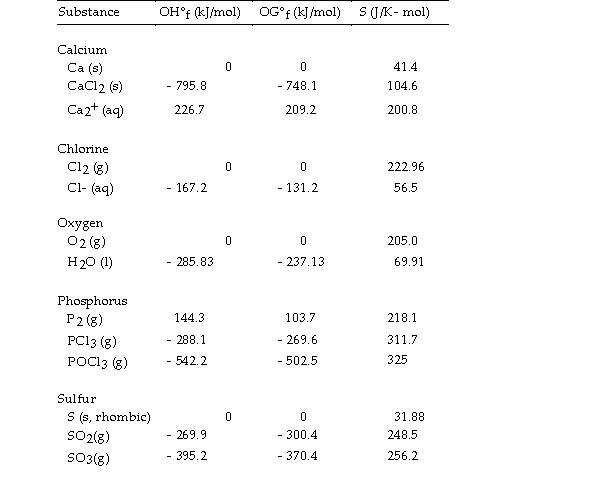
-The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
A) - 395.2
B) +395.2
C) - 790.4
D) +105.1
E) +790.4
Correct Answer:
Verified
Q41: Use the table below to answer the
Q42: Find the temperature above which a reaction
Q43: Use the table below to answer the
Q44: Use the table below to answer the
Q45: Calculate OG○(in kJ/mol) for the following reaction
Q47: Use the table below to answer the
Q48: Use the table below to answer the
Q49: Calculate OGo (in kJ/mol) for the following
Q50: Use the table below to answer the
Q51: For the reaction ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents