Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 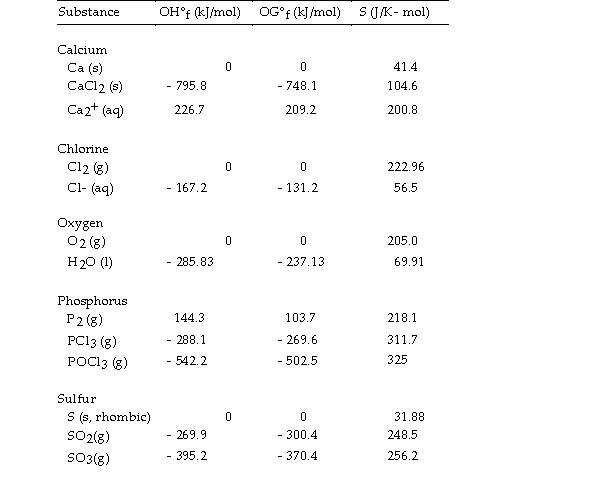
-The value of ΔG°at 25 °C for the decomposition of phosphorous trichloride into its constituent elements, 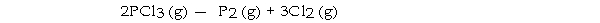 is kJ/mol.
is kJ/mol.
A) +642.9
B) - 373.3
C) - 539.2
D) - 642.9
E) +539.2
Correct Answer:
Verified
Q36: For a given reaction, ΔH = -
Q37: If ΔG° for a reaction is greater
Q38: Of the following, the entropy of is
Q39: Which reaction produces a decrease in the
Q40: Phosphorous and chlorine gases combine to produce
Q42: Find the temperature above which a reaction
Q43: Use the table below to answer the
Q44: Use the table below to answer the
Q45: Calculate OG○(in kJ/mol) for the following reaction
Q46: Use the table below to answer the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents