Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 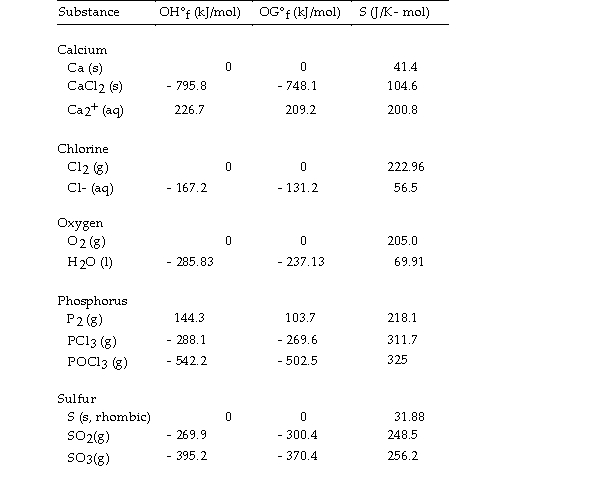
-Consider the reaction: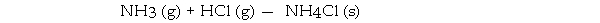
Given the following table of thermodynamic data at 298 oK:
The value of K for the reaction at 25 °C is .
A) 8.4 × 104
B) 1.4 × 108
C) 9.3 × 1015
D) 150
E) 1.1 × 10- 16
Correct Answer:
Verified
Q90: Use the table below to answer the
Q92: Use the table below to answer the
Q93: Use the table below to answer the
Q94: Use the table below to answer the
Q96: Use the table below to answer the
Q97: Use the table below to answer the
Q98: Use the table below to answer the
Q99: Use the table below to answer the
Q100: Use the table below to answer the
Q122: The melting of a substance at its
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents