Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 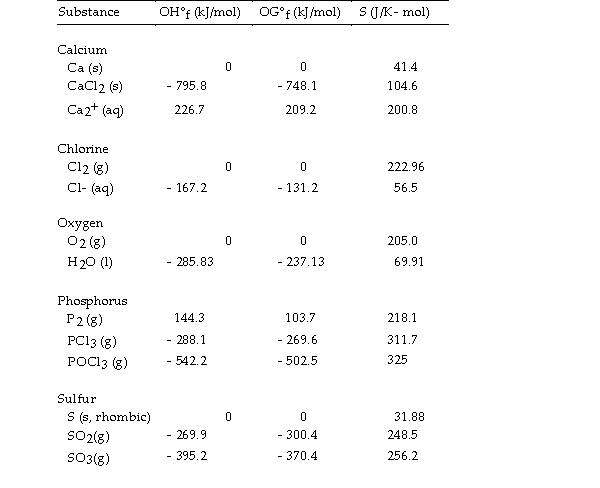
-The value of ΔG° at 373 K for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
A) +740.8
B) - 728.3
C) - 740.8
D) - 61.3
E) +61.3
Correct Answer:
Verified
Q89: Use the table below to answer the
Q90: Use the table below to answer the
Q93: Use the table below to answer the
Q94: Use the table below to answer the
Q95: Use the table below to answer the
Q96: Use the table below to answer the
Q97: Use the table below to answer the
Q122: The melting of a substance at its
Q124: The vaporization of a substance at its
Q125: The quantity of energy gained by a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents