A gas vessel is attached to an open- end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below.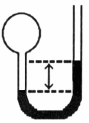
The difference in heights of the liquid in the two sides of the manometer is 32.3 cm when the atmospheric pressure is 765 mmHg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is atm.
A) 0.976
B) 1.08
C) 1.04
D) 1.01
E) 0.993
Correct Answer:
Verified
Q78: Using the van der Waals equation, the
Q79: The reaction of 50 mL of Cl2
Q80: The van der Waals equation for real
Q81: If 50.75 g of a gas occupies
Q82: A 0.325 L flask filled with gas
Q84: The pressure in a 12.2 L vessel
Q85: The volume of hydrogen gas at 38.0
Q86: A sample of gas (1.9 mol) is
Q87: A balloon originally had a volume of
Q88: A sample of He gas (2.0 mmol)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents