Which of the following statements is true concerning the voltaic cell shown below? 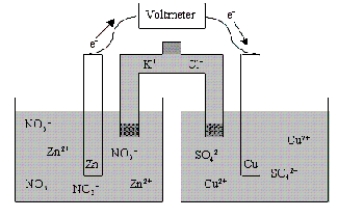
A) The Zn anode mass decreases as the cell discharges.
B) The Zn cathode mass increases as the cell discharges.
C) The Zn cathode mass decreases as the cell discharges.
D) The Zn anode mass increases as the cell discharges.
E) The mass of the Zn electrode neither increases nor decreases as the cell discharges.
Correct Answer:
Verified
Q3: Balance the following half-reaction occurring in an
Q13: Which of the following statements is true
Q16: Write a balanced half-reaction for the
Q17: In the context of the diagram given
Q19: Write a balanced net ionic equation
Q22: Given the following two half-reactions, write
Q25: Use the following standard reduction potentials
Q31: According to the cell notation below,which of
Q34: Consider the following half-reactions. Ag+(aq)+ e- →
Q38: Write a balanced net ionic equation for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents