Which of the following is FALSE about the energy diagram shown? 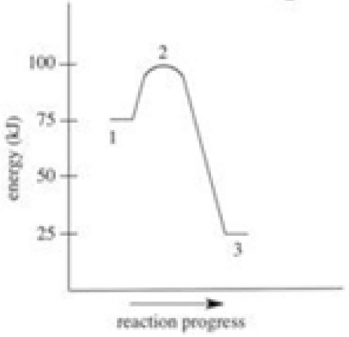
A) The activation energy is 100 kJ.
B) Point 2 on the diagram represents the activated complex.
C) This reaction is exothermic.
D) The energy of the products is lower than the energy of the reactants.
E) ∆H = −50 kJ
Correct Answer:
Verified
Q2: Which of the following statements concerning the
Q3: A carbohydrate sample weighing 0.235 g was
Q4: Which statement concerning energy changes in chemical
Q5: The rate of a chemical reaction increases
Q6: The amount of heat necessary to raise
Q7: Which of the following is always necessary
Q8: What instrument is used for measuring the
Q9: For the reaction shown below,Keq=2×1011.Which of the
Q10: The rate of a chemical reaction increases
Q11: What thermodynamic quantity is the ultimate predictor
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents