Glycerol is a very polar compound.Dimethyl ether is only slightly polar.Which statement is TRUE? 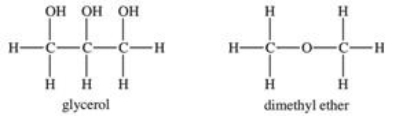
A) Glycerol is less viscous than dimethyl ether.
B) Glycerol will have a greater surface tension than dimethyl ether.
C) Dimethyl ether has a lower vapor pressure than glycerol.
D) The viscosity of both substances will increase with increasing temperature.
E) Glycerol will evaporate at a faster rate than dimethyl ether.
Correct Answer:
Verified
Q51: Which substance is expected to have the
Q52: How are the volume and temperature of
Q53: A balloon at 25°C has 1.00 L
Q54: Which of the following is a statement
Q55: A gas with pressure of 5.0 atm
Q57: What mass of helium is contained in
Q58: Which one of the following substances,with their
Q59: Surface tension
A)increases with increasing temperature.
B)is unaffected by
Q60: An aerosol spray paint can with a
Q61: Metals conduct electricity well due to the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents