Glycerol is a very polar compound.Dimethyl ether is only slightly polar.Which statement is TRUE? 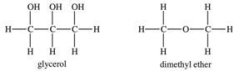
A) Glycerol is less viscous than dimethyl ether.
B) Glycerol will have a greater surface tension than dimethyl ether.
C) Dimethyl ether has a lower vapor pressure than glycerol.
D) The viscosity of both substances will increase with increasing temperature.
E) Glycerol will evaporate at a faster rate than dimethyl ether.
Correct Answer:
Verified
Q42: Which property is typical for molecular solids?
A)consist
Q43: Which of the following is TRUE for
Q46: What is the term that describes a
Q47: Which statement about a crystalline solid is
Q48: Of the following gases,which will behave most
Q49: Which statement is true concerning an ideal
Q52: How are the volume and temperature of
Q52: Which of the following is a statement
Q55: A gas with pressure of 5.0 atm
Q59: Surface tension
A)increases with increasing temperature.
B)is unaffected by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents