What can be said about the possibility of the existence of the hydrogen isotope represented by the symbol shown below? 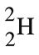
A) This isotope of hydrogen is not possible because it has no electrons.
B) This isotope of hydrogen is not possible because atoms of hydrogen have one proton.
C) This isotope of hydrogen is possible; it simply contains no protons and is an ion.
D) This isotope of hydrogen is possible; it simply contains no neutrons.
E) This isotope of hydrogen is possible; it simply has two neutrons.
Correct Answer:
Verified
Q62: Electron affinity is
A)the energy required to remove
Q64: Which of the following elements is most
Q67: Which of the following correctly gives the
Q68: Which of the following ions does not
Q70: Which of the following statements relating to
Q75: How many orbitals are there in the
Q76: How many sublevels are there in the
Q78: Which of the following atoms has the
Q92: Which statement is TRUE concerning Bohr's model
Q100: Rutherford's experiment,in which alpha particles were aimed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents