Which of the processes on a monatomic ideal gas given below will result in the temperature of the gas increasing? W is the work done by the gas.
A) a process where Q = W
B) a process where 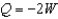
C) a process where Q = 2W
D) None of the above will increase the temperature of the gas.
Correct Answer:
Verified
Q2: In an isobaric process 3.6 * 104
Q2: A closed 2.0-L container holds 3.0 mol
Q3: A 4-mol ideal gas system undergoes an
Q3: According to the first law of thermodynamics,the
Q5: If an ideal gas does positive work
Q7: A 4-mol ideal gas system undergoes an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents