In the uranium isotope separation for 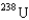 and
and 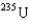 , the gas molecules used are
, the gas molecules used are 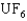 , uranium hexafluoride. The key to the separation is that in thermal equilibrium the average speed of the
, uranium hexafluoride. The key to the separation is that in thermal equilibrium the average speed of the 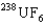 molecule is different from that of the
molecule is different from that of the 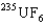 molecule. The fluorine atoms involved are all
molecule. The fluorine atoms involved are all 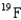 . Which of the hexafluoride molecules has the higher average speed and what is the ratio of the faster speed to the slower speed?
. Which of the hexafluoride molecules has the higher average speed and what is the ratio of the faster speed to the slower speed?
A) The 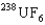 is the faster one. The ratio of its speed to that of
is the faster one. The ratio of its speed to that of 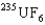 is 1.009.
is 1.009.
B) The 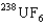 is the faster one. The ratio of its speed to that of
is the faster one. The ratio of its speed to that of 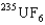 is 1.004.
is 1.004.
C) The 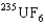 is the faster one. The ratio of its speed to that of
is the faster one. The ratio of its speed to that of 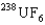 is 1.009.
is 1.009.
D) The 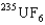 is the faster one. The ratio of its speed to that of
is the faster one. The ratio of its speed to that of 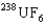 is 1.004.
is 1.004.
Correct Answer:
Verified
Q53: What is the internal energy of a
Q54: If the average kinetic energy of
Q55: For an ideal gas of a given
Q56: The internal energy of a monatomic ideal
Q57: The noble gases, listed by increasing molecular
Q59: If an average molecule diffuses to a
Q60: The absolute temperature of a monatomic ideal
Q61: Suppose an average molecule's displacement is 1.2
Q62: The ideal gas in a sealed container
Q82: If the temperature of an ideal gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents