Consider the reversible reaction: CO(g)+ Cl2(g) 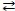 COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
COCl2(g). The reverse reaction is COCl2(g)→ CO(g)+ Cl2(g).
Correct Answer:
Verified
Q85: When reactants can come together and form
Q86: An enzyme contains a region called its
Q87: In the energy diagram shown below,C labels
Q88: In order to cause an endothermic equilibrium
Q89: When ΔH is negative,the bonds formed in
Q91: A manufacturing company requires 157 kJ of
Q92: When heat is added to an exothermic
Q93: Consider the reversible reaction: PCl3(g)+ Cl2(g)
Q94: As a general rule,a reaction rate _
Q95: In order to cause an endothermic equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents