The molecules A and B react according to the following chemical equation: A + B → C. Identify the limiting reactant under the reaction conditions shown in the molecular art below. 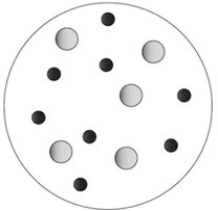 A = large grey circles B = small black circles
A = large grey circles B = small black circles
A) A is the limiting reactant
B) B is the limiting reactant
C) Both A and B are the limiting reactants
D) Neither A nor B are the limiting reactants
Correct Answer:
Verified
Q28: If a synthesis has four steps and
Q29: How many moles of sodium chloride (table
Q30: In the balanced redox reaction: 2 C2H6(g)+
Q31: How many moles of sulfur trioxide are
Q32: Suppose the theoretical yield in a reaction
Q34: In the balanced redox reaction: 2 C2H6(g)+
Q35: Which quantity has the greatest mass?
A)2.0 mol
Q36: Consider the reaction: 2 Al(OH)3 + 3
Q37: Potassium metal (K)reacts violently when added to
Q38: In the balanced redox reaction: 2 Cu(s)+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents