Does propane (shown) or octane (C8H18) exhibit stronger dispersion forces? 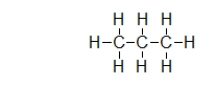
A) Propane.The smaller molecules can get closer to one another.
B) Propane.It has a larger permanent dipole.
C) Octane.It has a larger permanent dipole.
D) Octane.It has more electrons.
E) They both have the same amount of forces.
Correct Answer:
Verified
Q11: Which of the following covalent compounds is
Q12: Chloroform (CHCl3)is an anesthetic and is also
Q13: When determining the shape of a molecule,
Q14: Nitrogen trichloride, once used as a bleaching
Q15: What is the molecular geometry of the
Q17: How many electron groups do the carbon
Q18: Which of the molecules below exhibits hydrogen
Q19: How many nonbonding pairs are on the
Q20: Which of the following bonds is nonpolar?
A)
Q21: The electronegativity difference between C and H
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents