Which of the following covalent compounds is polar? 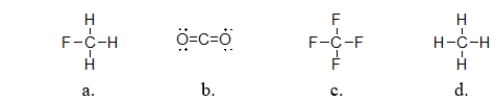
A) structure a
B) structure b
C) structure c
D) structure d
E) all of these molecules are polar.
Correct Answer:
Verified
Q6: What is the electron geometry of the
Q7: What is the molecular geometry of the
Q8: Which figure BEST illustrates how two molecules
Q9: Electrostatic interactions between positive and negative ions
Q10: An atom, X, has a tetrahedral electron
Q12: Chloroform (CHCl3)is an anesthetic and is also
Q13: When determining the shape of a molecule,
Q14: Nitrogen trichloride, once used as a bleaching
Q15: What is the molecular geometry of the
Q16: Does propane (shown)or octane (C8H18)exhibit stronger dispersion
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents