Does formaldehyde have a permanent dipole? 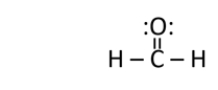
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The carbon is partially positive, and the oxygen is partially negative.
D) Yes.The carbon is partially positive, and the hydrogen is partially negative.
E) No.Formaldehyde only has a temporary dipole.
Correct Answer:
Verified
Q17: How many electron groups do the carbon
Q18: Which of the molecules below exhibits hydrogen
Q19: How many nonbonding pairs are on the
Q20: Which of the following bonds is nonpolar?
A)
Q21: The electronegativity difference between C and H
Q23: What is the molecular geometry of the
Q24: Which element is the MOST electronegative?
A) fluorine
B)
Q25: A polar molecule is one that has
A)
Q26: In molecules with permanent dipoles, _ are
Q27: What is the strongest type of intermolecular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents