What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with other caffeine molecules? 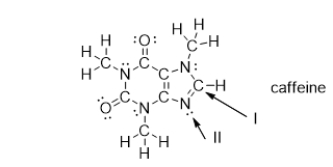
A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions
Correct Answer:
Verified
Q22: Does formaldehyde have a permanent dipole?
Q23: What is the molecular geometry of the
Q24: Which element is the MOST electronegative?
A) fluorine
B)
Q25: A polar molecule is one that has
A)
Q26: In molecules with permanent dipoles, _ are
Q28: How does VSEPR theory explain the electron
Q29: Why do electron groups around a central
Q30: What is the strongest type of intermolecular
Q31: Atom X in a molecule has tetrahedral
Q32: Is chloroform (CHCl3)a polar molecule?
A) No.All polarities
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents