The structure below is a Lewis structure of methane.This Lewis structure tells us many things about methane that are useful.However, it also suggests one characteristic of methane that is, in fact, false.What is this one characteristic? 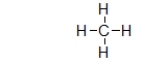
A) The Lewis structure suggests that carbon has a full valence shell, but it really doesn't.
B) The Lewis structure suggests that carbon has four bonds, but it really doesn't.
C) The Lewis structure suggests that carbon is bonded to four hydrogens, but it really isn't.
D) The Lewis structure suggests that methane is flat, but it really isn't.
E) The Lewis structure suggests that methane is stable, but it really isn't.
Correct Answer:
Verified
Q76: _ is the sharing of electrons between
Q77: How many atoms are bonded to the
Q78: What is the angle between groups of
Q79: Which of the bonds in the following
Q80: What is the molecular geometry of nitrogen
Q81: What is the electron geometry of the
Q82: What is meant by the following symbols?
Q84: How is estradiol recognized by the estrogen
Q85: What is the electron geometry of each
Q86: What is the molecular geometry of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents