The isoelectric pH (pI) of 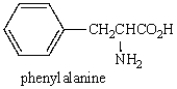 is likely to be at a pH that is
is likely to be at a pH that is
A) below pK1.
B) at pK1.
C) between pK1 and pK2.
D) at pK2.
E) above pK2.
Correct Answer:
Verified
Q32: Sanger's reagent is used
A) to determine the
Q33: Based on the equilibria shown below, what
Q34: Which reagent is used specifically to cleave
Q35: The Edman degradation is used
A) to identify
Q36: Ninhydrin is used
A) to determine the N-terminal
Q38: What is the structure of the phenylhydantoin
Q39: A tetrapeptide contains the amino acids Phe,
Q40: The mechanism by which Sanger's reagent reacts
Q41: The reaction of Q42: Sanger's reagent (2,4-dinitrofluorobenzene) reacts with the following![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents