The formal charges in the perchlorate ion are: 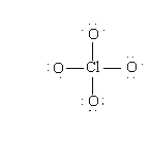
A) -1 on each O and +3 on the Cl.
B) 0 on each O and -1 on the Cl.
C) -1 on each O and +4 on the Cl.
D) -1/4 on each O and 0 on the Cl.
E) +1 on each O and -1 on the Cl.
Correct Answer:
Verified
Q19: Which of the following elements has three
Q20: The most electronegative elements in the periodic
Q21: Which of the following molecules is heterocyclic?
A)
Q22: Which of the following molecules can be
Q23: The curved arrows in the resonance structure
Q25: What is the percent s character in
Q26: What is the formal charge of N
Q27: The formal charges in the complex
Q29: The maximum number of electrons that a
Q30: For carbon monoxide, ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents