The formal charges in the complex 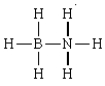 are:
are:
A) 0 on each H, +1 on N, and -1 on B.
B) +1 on each H, +1 on N, and -1 on B.
C) 0 on each H, -1 on N, and +1 on B.
D) 0 on each H, 0 on N, and 0 on B.
E) -1 on each H, +3 on N, and +3 on B.
Correct Answer:
Verified
Q22: Which of the following molecules can be
Q23: The curved arrows in the resonance structure
Q24: The formal charges in the perchlorate ion
Q25: What is the percent s character in
Q26: What is the formal charge of N
Q29: The maximum number of electrons that a
Q30: For carbon monoxide, Q30: For carbon monoxide, Q31: The structural formula Q32: Which of the following molecules is acyclic? Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
A)

