In the energy level diagram shown in the figure below, the electron is excited to the E2 energy level.If the atom absorbs a photon with the exact frequency to move the electron to another energy level, which energy level would correspond to the incoming photon with the largest frequency? 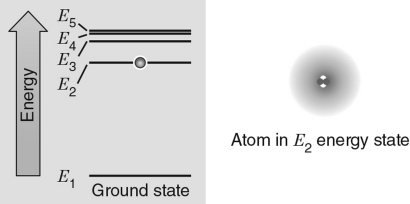
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:
Verified
Q33: As wavelength increases, the energy of a
Q34: The Doppler shift can be used to
Q35: If you observe the spectrum of an
Q36: In the energy level diagram shown in
Q37: A red photon has a wavelength of
Q39: If you observe the spectrum of a
Q40: Why is a neutral iron atom a
Q41: Compare two blackbody objects, one at 200
Q42: You observe the spectrum of two stars.Star
Q43: If the quantity of something entering a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents