In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest energy? 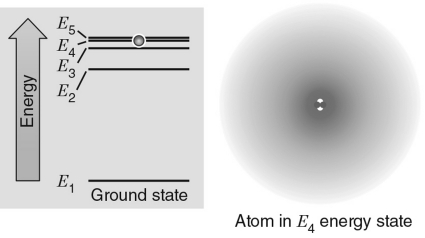
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:
Verified
Q31: In the energy level diagram shown in
Q32: In the quantum mechanical view of the
Q33: As wavelength increases, the energy of a
Q34: The Doppler shift can be used to
Q35: If you observe the spectrum of an
Q37: A red photon has a wavelength of
Q38: In the energy level diagram shown in
Q39: If you observe the spectrum of a
Q40: Why is a neutral iron atom a
Q41: Compare two blackbody objects, one at 200
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents