In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest wavelength? 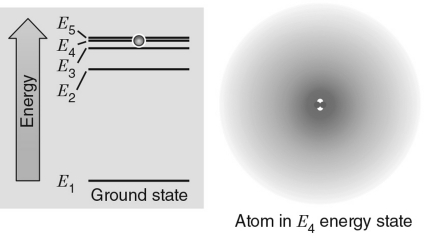
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:
Verified
Q26: When an electron moves from a higher
Q27: The figure below illustrates a stellar spectrum.The
Q28: In the energy level diagram shown in
Q29: The frequency of a wave is
A) the
Q30: Light with a wavelength of 600 nm
Q32: In the quantum mechanical view of the
Q33: As wavelength increases, the energy of a
Q34: The Doppler shift can be used to
Q35: If you observe the spectrum of an
Q36: In the energy level diagram shown in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents