In the energy level diagram shown in the figure below, the electron is excited to the E4 energy level.If the electron transitions to an energy level giving off a photon, which level would produce a photon with the largest frequency? 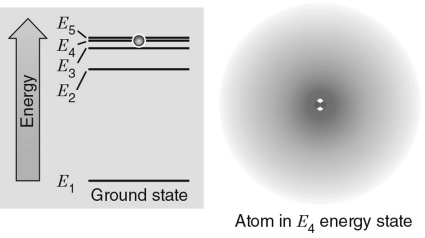
A) E1
B) E2
C) E3
D) E4
E) E5
Correct Answer:
Verified
Q23: If the frequency of a beam of
Q24: Which of these objects would emit an
Q25: The n = 5 electronic energy level
Q26: When an electron moves from a higher
Q27: The figure below illustrates a stellar spectrum.The
Q29: The frequency of a wave is
A) the
Q30: Light with a wavelength of 600 nm
Q31: In the energy level diagram shown in
Q32: In the quantum mechanical view of the
Q33: As wavelength increases, the energy of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents