A 810-g quantity of ethanol, in the liquid state at its melting point of 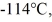 is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol ∙ K) . The change in the entropy of the ethanol as it freezes is closest to
is frozen at atmospheric pressure. The heat of fusion of ethanol is 1.04 × 105 J/kg, the molecular mass is 46.1 g/mol, and the ideal gas constant is R = 8.314 J/(mol ∙ K) . The change in the entropy of the ethanol as it freezes is closest to
A) -540 J/K.
B) -490 J/K.
C) -600 J/K.
D) 490 J/K.
E) 540 J/K.
Correct Answer:
Verified
Q26: What is the maximum theoretical efficiency possible
Q27: A Carnot refrigerator takes heat from water
Q35: A 610-g quantity of an ideal gas
Q37: What is the change in entropy of
Q40: A Carnot engine is operated as a
Q40: A Carnot engine operates between reservoirs at
Q41: A brass rod, 75.0 cm long and
Q42: At atmospheric pressure, 45 moles of liquid
Q43: A 2.00-kg block of ice at 0.00°C
Q44: A system consists of two very large
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents