Dust particles are pulverized rock, which has density 2500 kg/m3. They are approximately spheres 20 μm in diameter. Treating dust as an ideal gas, what is the root-mean-square speed (thermal speed) of a dust particle at 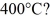 (The Boltzmann constant is 1.38 × 10-23 J/K.)
(The Boltzmann constant is 1.38 × 10-23 J/K.)
A) 5.2 × 10-5 m/s
B) 1.7 × 10-5 m/s
C) 3.0 × 10-5 m/s
D) 7.3 × 10-5 m/s
Correct Answer:
Verified
Q21: The mean free path of an oxygen
Q24: What is the average kinetic energy of
Q26: Assuming the radius of diatomic molecules is
Q28: An oxygen molecule falls in a vacuum.From
Q28: A 0.10- Q29: The root-mean-square speed (thermal speed)of the molecules Q35: A sealed container holds 0.020 moles of Q42: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the Q48: At 50.0°C,the average translational kinetic energy of Q53: The root-mean-square speed (thermal speed)of the molecules![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents