If you add 700 kJ of heat to 700 g of water at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 2.26 × 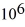 J/kg and its specific heat is 4190 J/(kg ∙ K) .
J/kg and its specific heat is 4190 J/(kg ∙ K) .
A) 429 g
B) 258 g
C) 340 g
D) 600 g
E) none
Correct Answer:
Verified
Q25: If 2.0 g of water at 0.00°C
Q30: A 200-g metal container, insulated on the
Q30: A 406.0 kg copper bar is put
Q31: A substance has a melting point of
Q32: An 80-g aluminum calorimeter contains 380 g
Q35: Two metal rods, one silver and the
Q36: A 400-g piece of metal at 120.0°C
Q39: A substance has a melting point of
Q40: Two experimental runs are performed to determine
Q41: A solid concrete wall 4.0 m by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents