An 80-g aluminum calorimeter contains 380 g of water at an equilibrium temperature of 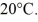 A
A 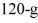 piece of metal, initially at
piece of metal, initially at 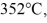 is added to the calorimeter. The final temperature at equilibrium is 32°C. Assume there is no external heat exchange. The specific heats of aluminum and water are 910 J/kg ∙ K and 4190 J/kg ∙ K, respectively. The specific heat of the metal is closest to
is added to the calorimeter. The final temperature at equilibrium is 32°C. Assume there is no external heat exchange. The specific heats of aluminum and water are 910 J/kg ∙ K and 4190 J/kg ∙ K, respectively. The specific heat of the metal is closest to
A) 520 J/kg ∙ K.
B) 480 J/kg ∙ K.
C) 390 J/kg ∙ K.
D) 350 J/kg ∙ K.
E) 560 J/kg ∙ K.
Correct Answer:
Verified
Q30: A 200-g metal container, insulated on the
Q30: A 406.0 kg copper bar is put
Q31: A substance has a melting point of
Q33: If you add 700 kJ of heat
Q35: Two metal rods, one silver and the
Q36: A 400-g piece of metal at 120.0°C
Q39: A substance has a melting point of
Q40: Two experimental runs are performed to determine
Q41: A solid concrete wall 4.0 m by
Q56: A copper cylinder with a mass of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents