The following nuclear reaction releases energy. Which has more mass, the reactants or the products? 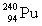
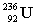 +
+ 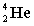 + energy
+ energy
A) neither because of the law of conservation of mass
B) the reactant because some of the mass is converted to energy
C) the products because some of the energy is converted to mass
D) the products because some of the mass is converted to energy
Correct Answer:
Verified
Q42: The most stable isotope is iron-56.What kind
Q44: The control rods in a nuclear reactor
Q45: Complete the fission reaction of plutonium-239:
Q47: Complete the following fusion reaction.
_
Q47: The reaction in which neutrons from the
Q48: The following nuclear reaction absorbs energy.
Q48: The sequence of energy changes for a
Q49: Complete the fission reaction of plutonium-239:
Q50: Complete the fission reaction of plutonium-239:
Q51: Complete the following fusion reaction.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents