In the diagram, the forward reaction will probably not proceed without an input of energy because the: 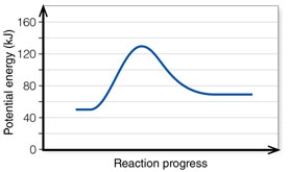
A) activation energy is too high.
B) net energy change is too low.
C) products are too far apart from the reactants.
D) reactant energy is too high to start with.
Correct Answer:
Verified
Q1: In the diagram, the number 1 corresponds
Q2: In the diagram, the activation energy of
Q4: In the diagram, the number 3 corresponds
Q6: In the diagram, the energy of the
Q6: Which statement correctly describes reaction rates?
A) Decreasing
Q7: In the diagram, how do the concentrations
Q9: In the diagram, the FORWARD reaction is:
Q11: In the diagram, the net energy change
Q15: Chemical reactions occur when molecules,atoms,or ions collide.However,not
Q20: Which factor tends to increase the rate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents