In the diagram, how do the concentrations of the reactants and products compare at equilibrium as a general rule? 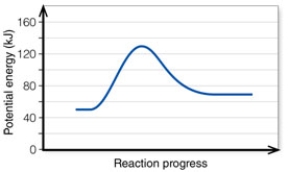
A) They are equal.
B) The reactant concentration is greater than that of the product.
C) The product concentration is greater than that of the reactant.
Correct Answer:
Verified
Q2: In the diagram, the activation energy of
Q3: In the diagram, the forward reaction will
Q4: In the diagram, the number 3 corresponds
Q6: In the diagram, the energy of the
Q6: Which statement correctly describes reaction rates?
A) Decreasing
Q9: In the diagram, the FORWARD reaction is:
Q11: In the diagram, the net energy change
Q12: In the diagram, the reaction described is
Q15: Chemical reactions occur when molecules,atoms,or ions collide.However,not
Q20: Which factor tends to increase the rate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents