Write the solubility product expression for the following solubility equilibrium: BaSO4 (s) Ba2+ (aq) + SO42- (aq)
A) 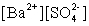
B) 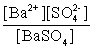
C) 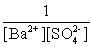
D) 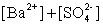
Correct Answer:
Verified
Q22: The equilibrium expression of the following
Q29: Which K value indicates a reaction that
Q30: The symbol used to represent the equilibrium
Q31: In a saturated solution of aluminum phosphate,AlPO4,both
Q32: Consider the equilibrium reaction between the
Q33: In a saturated solution of cobalt(II)carbonate,CoCO3,both [Co2+]
Q37: Given the following equation and equilibrium
Q38: According to Le Châtelier's principle:
A) equilibrium cannot
Q39: Which of the following K values indicates
Q40: Cyanic acid,HOCN,ionizes in water to produce
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents