The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3. Find the value of volume V3. The atomic mass of helium is 4.0 g/mol, and R = 8.31 J/mol ∙ K. 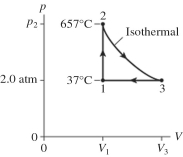
A) 25 L
B) 99 L
C) 50 L
D) 12 L
Correct Answer:
Verified
Q64: How much heat is required to increase
Q82: A gas expands from an initial volume
Q205: The temperature of an ideal gas
Q206: The figure shows a pV diagram for
Q207: A compression at a constant pressure
Q208: A rigid container is filled with
Q209: A sealed 87-
Q211: The temperature of an ideal gas
Q212: The figure shows a pV diagram for
Q214: An expansion process on an ideal
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents