The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a) pressure p1 and (b) temperature T2? 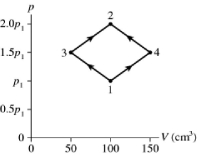
A) (a) 81 atm, (b) 660°C
B) (a) 14 atm, (b) 660°C
C) (a) 81 atm, (b) 120°C
D) (a) 14 atm, (b) 120°C
Correct Answer:
Verified
Q62: A container with rigid walls is filled
Q65: Dust particles in a grain elevator frequently
Q71: If the temperature of an ideal gas
Q72: What is the average translational kinetic energy
Q80: At what temperature is the rms speed
Q198: (a) At what Celsius temperature is the
Q203: The figure shows a pV diagram
Q204: The figure shows a pV diagram for
Q205: The temperature of an ideal gas
Q206: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents