The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container. The temperature of state 1 is 76° C, the atomic mass of the oxygen atom is 16 g/mol, and R = 8.31 J/mol ∙ K. What are the temperatures T3 and T4? 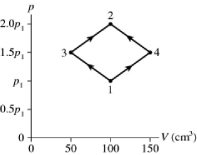
A) -11° C and 510° C
B) 57° C and 170° C
C) 260° C and 790° C
D) 38° C and 110° C
Correct Answer:
Verified
Q62: A container with rigid walls is filled
Q72: What is the average translational kinetic energy
Q80: At what temperature is the rms speed
Q201: The figure shows a pV diagram for
Q203: The figure shows a pV diagram
Q205: The temperature of an ideal gas
Q206: The figure shows a pV diagram for
Q207: A compression at a constant pressure
Q208: A rigid container is filled with
Q209: A sealed 87-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents