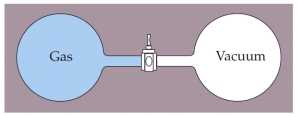
 Two containers of equal volume are connected by a stopcock as shown above. One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum. The containers are thermally isolated from the surroundings so no heat enters or escapes from the system. The stopcock is then opened allowing the gas from one container to fill the other. What is the final temperature of the gas after it has come to equilibrium?
Two containers of equal volume are connected by a stopcock as shown above. One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum. The containers are thermally isolated from the surroundings so no heat enters or escapes from the system. The stopcock is then opened allowing the gas from one container to fill the other. What is the final temperature of the gas after it has come to equilibrium?
A) 136.5 K
B) 273 K
C) 293 K
D) 195 K
E) undetermined
Correct Answer:
Verified
Q3: The first law of thermodynamics has as
Q5: In physics, we typically write the first
Q6: You exercise on a stationary bike and
Q7: There is no heat transfer into or
Q44: In a certain thermodynamic process,1000 cal of
Q50: The first law of thermodynamics is most
Q54: The percentage of mechanical energy that can
Q56: How much internal energy is contained in
Q57: A system absorbs heat Q and has
Q59: During a certain thermodynamic process,418 J of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents