Consider the two containers below, which are separated by a semipermeable membrane that only allows the passage of small solutes. If A contains 6% NaCl in water and B contains 3% NaCl in water, at what point will diffusion stop? 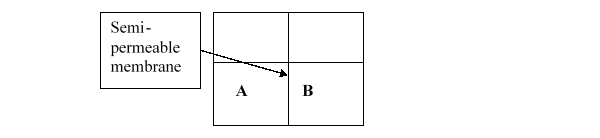
A) When the volume of water in B is twice as large as the volume of water in C.
B) When there is no water left in A.
C) When there is no water left in B.
D) When the volume of water in A is twice as large as the volume of water in B.
E) When the concentration of NaCl is equal in A and B.
Correct Answer:
Verified
Q24: A person receiving IV fluids must always
Q83: Consider the two containers below, which are
Q84: Consider solutions A and B separated by
Q85: Which term best describes the process illustrated
Q87: Red blood cells are placed into three
Q88: Consider solutions A and B separated by
Q89: The function of a kidney is similar
Q90: The function of dialysate is similar in
Q92: Which of the following statements explains why
Q93: Red blood cells are placed into three
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents